41 fda health claims on food labels
The Basics of Food Product Health Claims - LabelCalc For additional information from the FDA on Health Claims, Download this PDF and scroll to Appendix C. Claim #3: Qualified Health Claims. Qualified food product health claims refer to a claim made on a food product label in reference to a disease that does not have to meet the rigorous standards of an Authorized Health Claim. › milestones-us-food-and-drug-lawMilestones in U.S. Food and Drug Law | FDA The law preempts state requirements about food standards, nutrition labeling, and health claims and, for the first time, authorizes some health claims for foods. The food ingredient panel, serving ...
Questions and Answers on Health Claims in Food Labeling | FDA Health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products to show that a food or food component may reduce the risk of a disease or a...

Fda health claims on food labels
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... FDA proposes voluntary 'healthy' food label claim However, foods must meet specific nutrient-related criteria to use the nutrient content claim "healthy.". On September 28, 2022, the FDA issued a proposed rule to update the definition of the nutrient content claim "healthy," which was set in 1994. The existing definition has limits for total fat, saturated fat, cholesterol and sodium ... › food › food-labeling-nutritionStructure/Function Claims | FDA - U.S. Food and Drug ... Mar 07, 2022 · Final Rule: Food Labeling: Nutrient Content Claims, Health Claims, and Statements of Nutritional Support for Dietary Supplements (62 Fed. Reg. 49859 at 49863-49866) Conventional Foods
Fda health claims on food labels. What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims) Everything you need to know about Health Claims on Food Labels The authorized health claims by the FDA must have significant scientific agreement among qualified experts to support the scientific evidence for a substance - disease relationship. The FDA has approved 12 health claims on food labels such as sodium and hypertension; fiber-containing grains, fruits and vegetables and cancer; and more. › food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ... › food › food-labeling-nutritionLabel Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
FDA Proposes Updated Definition of 'Healthy' Claim on Food Packages to ... Today, the U.S. Food and Drug Administration proposed updated criteria for when foods can be labeled with the nutrient content claim "healthy" on their packaging. This proposed rule would... › food › food-labeling-nutritionQuestions and Answers on Dietary Fiber | FDA - U.S. Food and ... Dec 17, 2021 · Also, if an added isolated or synthetic non-digestible carbohydrate is the subject of an authorized health claim that FDA has previously evaluated using the health claim petition process in 21 CFR ... › animal-veterinary › animal-food-feedsPet Food | FDA - U.S. Food and Drug Administration For more information about labeling requirements, see Pet Food Labels - General. FDA also reviews specific claims on pet food, such as “maintains urinary tract health,” “low magnesium ... Use of the Term Healthy on Food Labeling | FDA The FDA has begun a public process to update the "healthy" claim for food labeling to be consistent with current nutrition science and federal dietary guidance. Updating the "healthy" claim...
Food Label Claims: What You Can and Can't Trust - WebMD Food Claims to Watch Out For. Some health claims on foods lack official definitions. ... FDA: "Label Claims for Conventional Foods and Dietary Supplements," "Organic on Food Labels," "Producing a ... FDA Proposes to Update Definition for "Healthy" Claim on Food Labels The "healthy" claim can act as a quick signal on food package labels to help empower consumers, including those with lower nutrition knowledge, with information to identify foods that will... A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." FDA Permits Qualified Health Claims on Nut Labels For the first time, the U.S. Food and Drug Administration has recognized a qualified health claim made by a conventional food. In a surprise move in July the FDA granted permission for peanuts, almonds, hazelnuts, pecans, pistachios and walnuts to carry a label touting their heart-healthy effects.
FDA perspectives on health claims for food labels - PubMed FDA perspectives on health claims for food labels Authors J Craig Rowlands 1 , James E Hoadley Affiliation 1 Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, College Park, MD 20740, USA. JCRowlands@Dow.com PMID: 16480811 DOI: 10.1016/j.tox.2005.10.023 Food Labeling* Legislation, Food* Nutritive Value Research Design
Health Claims On Food Labels Health Claims On Food Labels PubMed. Health Health claims on food labels Mil Med. 1994 Mar;159(3):213-7. Author L Tollefson 1 Affiliation 1 Food and Drug Administration, Center for Food Safety and Applied Nutrition, Washington, DC … Detail: Visit URL . Category: Health View Health
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written...
Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and
Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are...
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...
› food › food-labeling-nutritionStructure/Function Claims | FDA - U.S. Food and Drug ... Mar 07, 2022 · Final Rule: Food Labeling: Nutrient Content Claims, Health Claims, and Statements of Nutritional Support for Dietary Supplements (62 Fed. Reg. 49859 at 49863-49866) Conventional Foods
FDA proposes voluntary 'healthy' food label claim However, foods must meet specific nutrient-related criteria to use the nutrient content claim "healthy.". On September 28, 2022, the FDA issued a proposed rule to update the definition of the nutrient content claim "healthy," which was set in 1994. The existing definition has limits for total fat, saturated fat, cholesterol and sodium ...
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and...




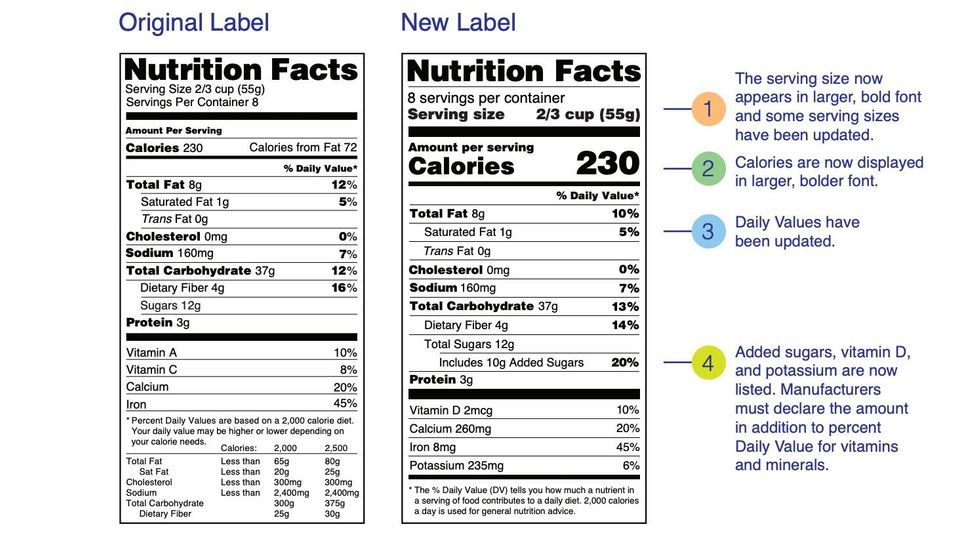




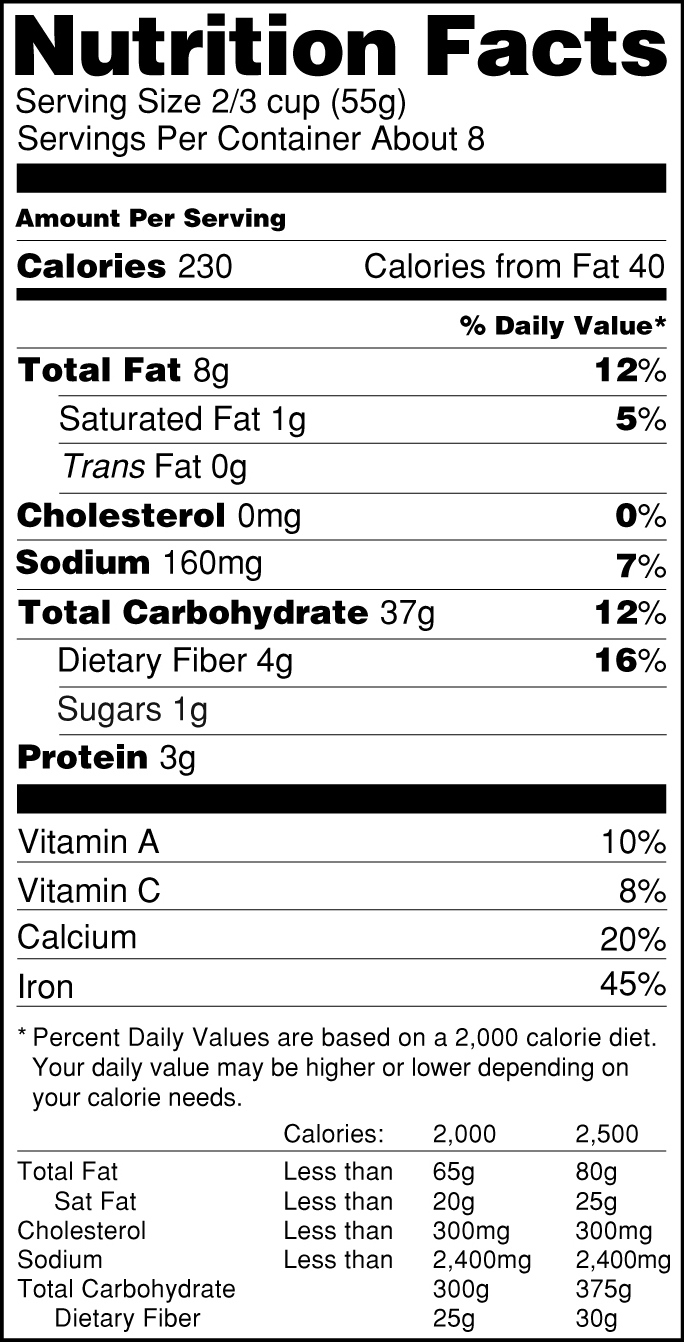

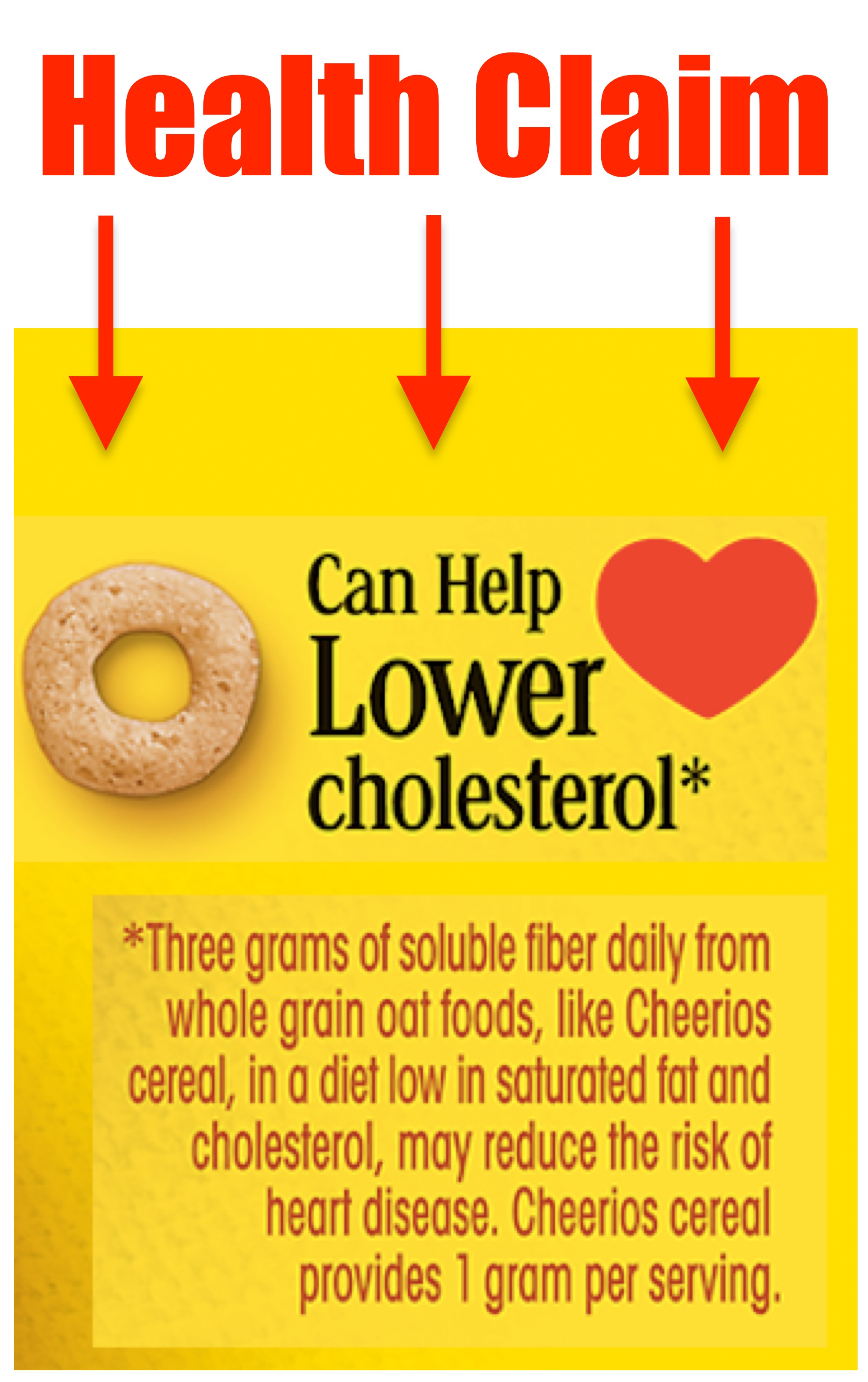














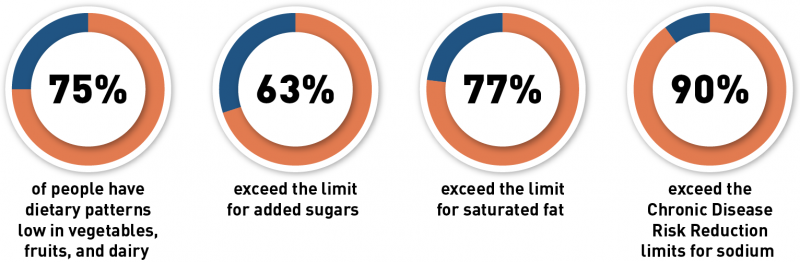







Post a Comment for "41 fda health claims on food labels"