40 medication labels must include
Labeling Information | Drug Products | FDA WebFor more information on labeling, including Physician Labeling Rule (PLR) requirements, guidances, presentations, sample templates and format tools, and established … FDA’s Labeling Resources for Human Prescription Drugs | FDA Web08.08.2022 · Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes …
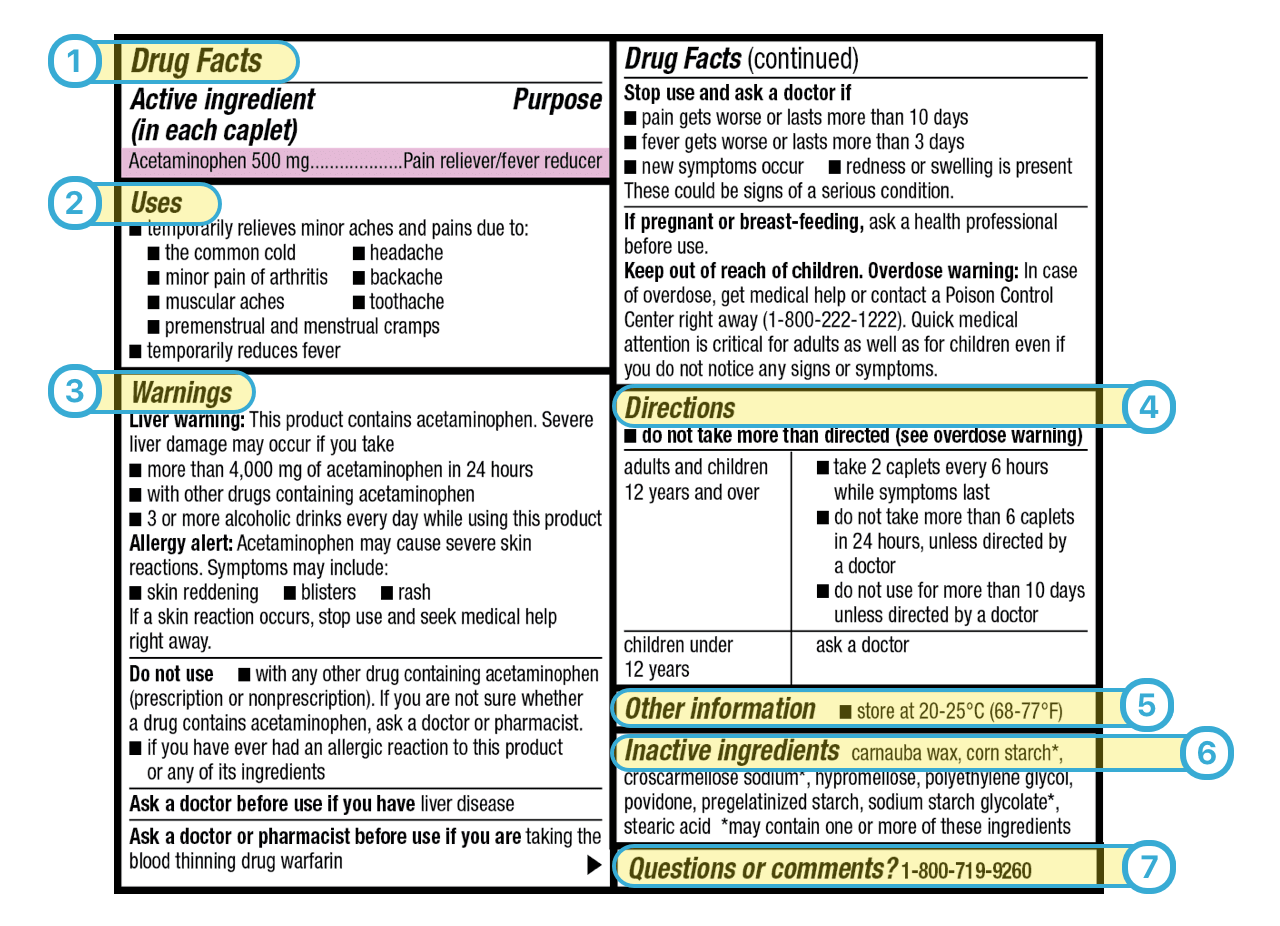
The Over-the-Counter Medicine Label: Take a Look | FDA WebAll nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an …

Medication labels must include
Medication labels Flashcards | Quizlet WebBy federal law drug labels must contain the following information: manufacturer name, expiration date, control numbers, a barcode, and a code identifying one of the two official … Pharmaceutical Labeling: Requirements & Guidelines Web19.07.2020 · All drug products must be registered with the FDA, have a National Drug Code (NDC), and have that three-section NDC code printed on the front of the label. (You can read more about the NDC here.) Breaking … Drug labeling, Information about Drug labeling - FAQs Web01.06.1999 · The label must include instructions for correctly using the medicine, including the dosage and what to do if the patient misses a dose. This description should cover …
Medication labels must include. Medicines: packaging, labelling and patient information … Web18.12.2014 · Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional warning statements … Chapter 5: Prescriptions and Labels Flashcards | Quizlet WebRegulated by the Food and Drug Administration (FDA), which determines what needs to be on the label Dispensing pharmacist's label must include: Pharmacy name, address, and … What's on my medicine label? - Therapeutic Goods Administration … Best practice in the labelling and packaging of medicines Web29.12.2014 · As part of a move towards an increase in self-regulation of medicines labelling and packaging, this guidance has been developed to aid those responsible for the …
Drug labeling, Information about Drug labeling - FAQs Web01.06.1999 · The label must include instructions for correctly using the medicine, including the dosage and what to do if the patient misses a dose. This description should cover … Pharmaceutical Labeling: Requirements & Guidelines Web19.07.2020 · All drug products must be registered with the FDA, have a National Drug Code (NDC), and have that three-section NDC code printed on the front of the label. (You can read more about the NDC here.) Breaking … Medication labels Flashcards | Quizlet WebBy federal law drug labels must contain the following information: manufacturer name, expiration date, control numbers, a barcode, and a code identifying one of the two official …

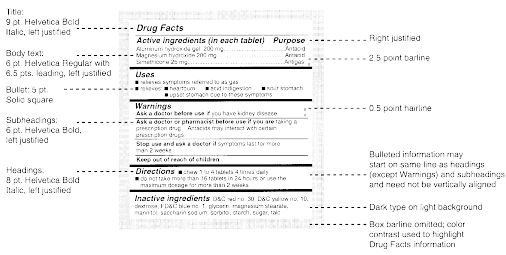

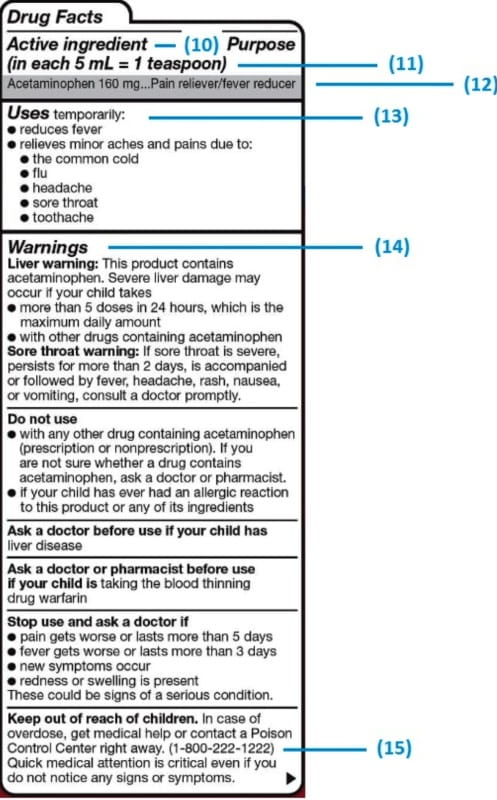




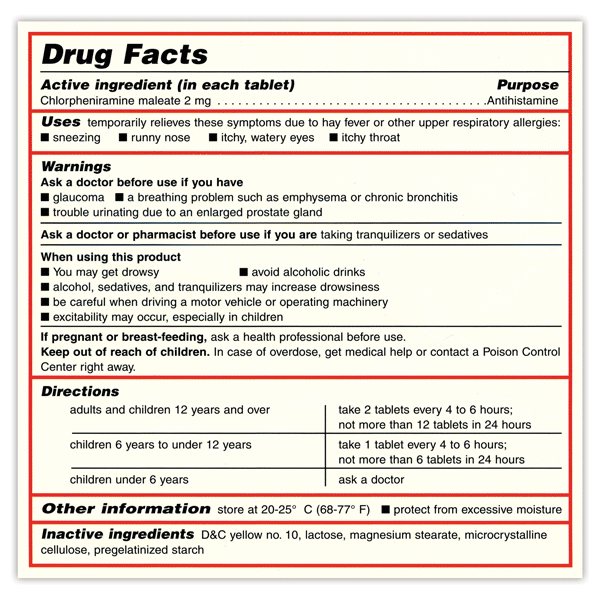

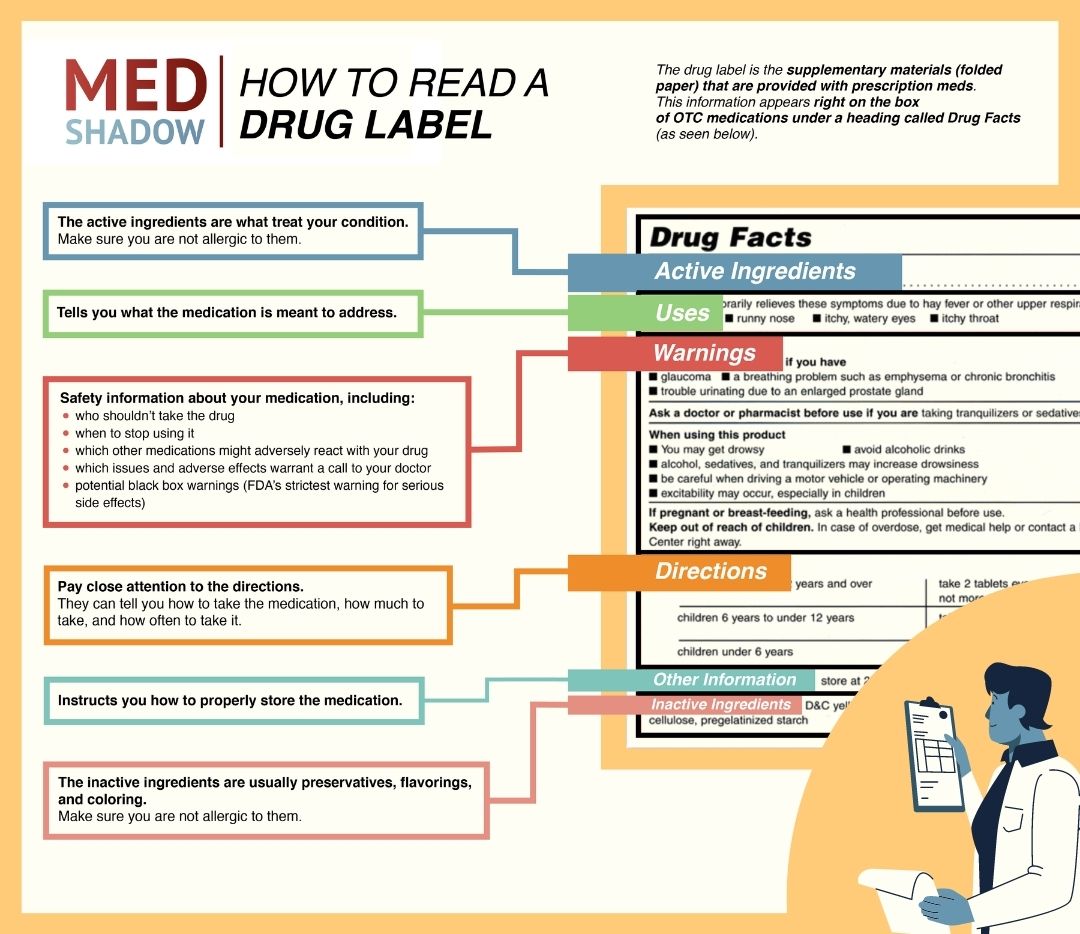


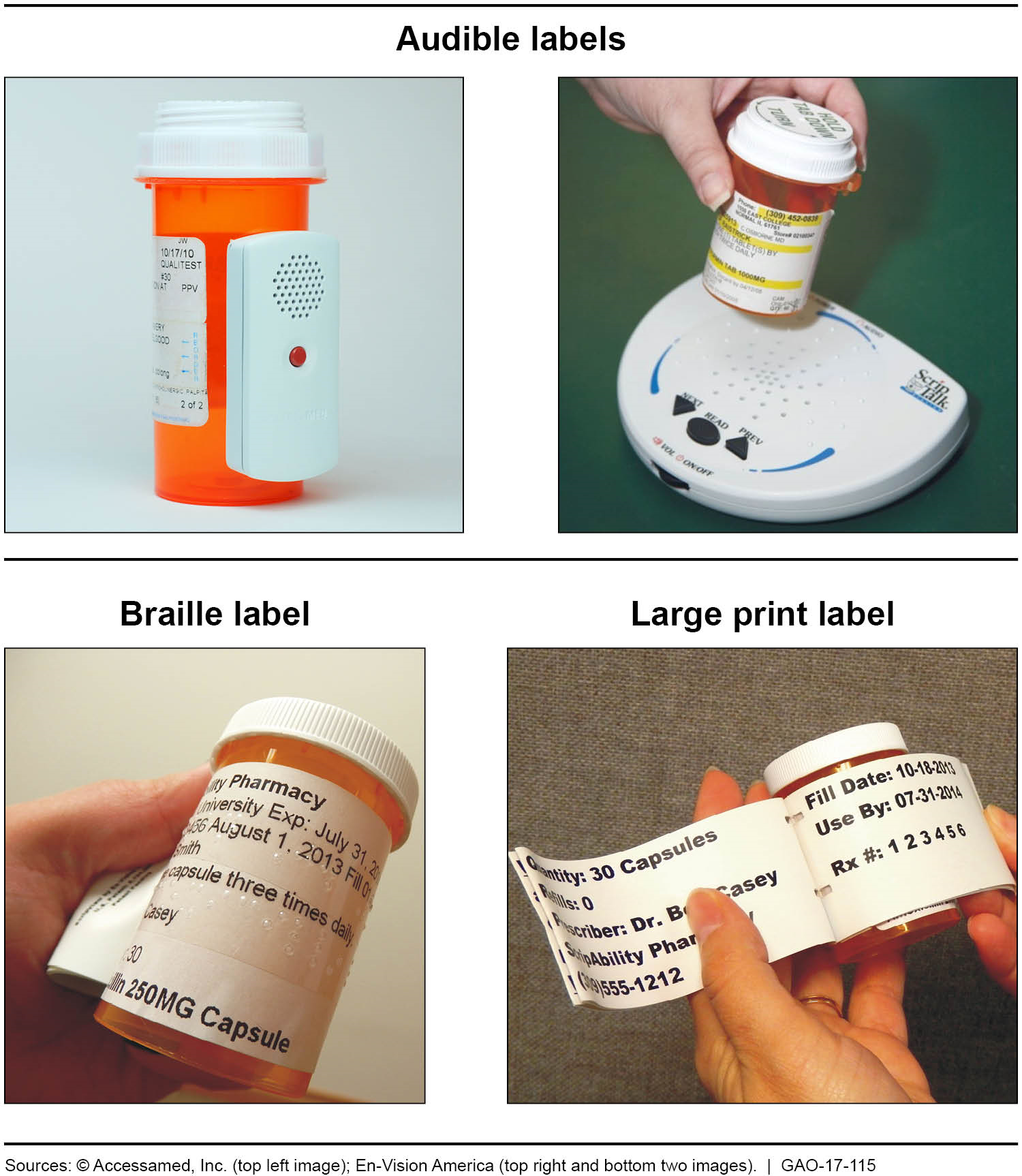



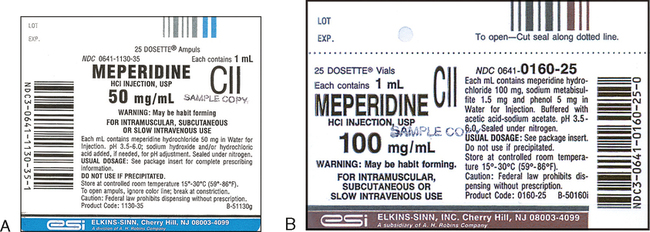






Pharmaceutical quality assurance companies often work closely with pharmaceutical manufacturers to implement best practices in labeling. This involves staying abreast of industry developments, incorporating the latest technologies, and ensuring that labeling processes align with the highest standards.
ReplyDelete